COPD
- pathophysio
a preventable and treatable respiratory disorder largely caused by smoking, is characterised by progressive, partially reversible airflow obstruction and lung hyperinflation with significant extrapulmonary (systemic) manifestations1 (Level II-2) and comorbid conditions2 (Level II-3) all of which may contribute to the severity of the disease in individual patients.
due to a mixture of small airway disease (obstructive bronchiolitis) and lung parenchymal destruction (emphysema), the relative contributions of which vary from individual to individual. Airflow limitation, associated with an abnormal inflammatory reaction of the lung to noxious particles or gases, the most common of which worldwide is cigarette smoke, is usually progressive, especially if exposure to the noxious agents persists.
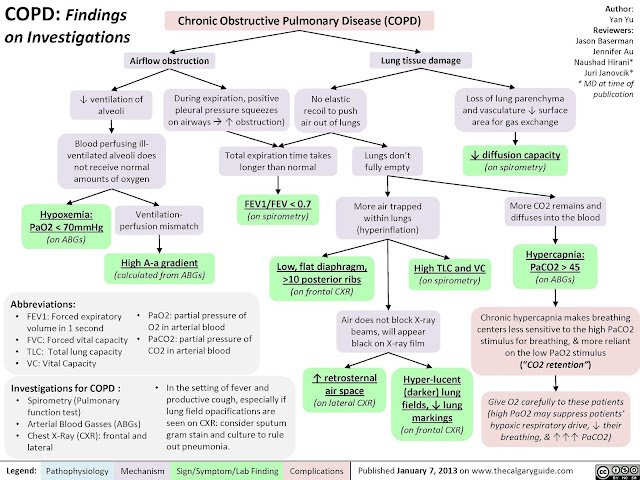
diagnosis and severity assessment of COPD, a post-bronchodilator FEV1 /FVC ratio of < 0.70 and post-bronchodilator FEV1 measurement, respectively are recommended.
The pathological changes in COPD,
which include chronic inflammation and structural changes resulting from repeated injury and repair - -
- due to inhaled cigarette smoke and other noxious particles,
- are found in the proximal airways, peripheral airways, lung parenchyma, and pulmonary vasculature.
The chronic inflammation in COPD is
- characterised by
- an increase in the numbers of neutrophils (in the airway lumen),
- macrophages (in the airway lumen, airway wall, and parenchyma), and
- CD8+ lymphocytes (in the airway wall and parenchyma).
- differences in physiological changes, symptoms and response to treatment
- These pathological changes lead to
- mucus hypersecretion,
- expiratory airflow limitation
- with dynamic small airway collapse causing
- air trapping and lung hyperinflation,
- gas exchange abnormalities, and
- progressive pulmonary hypertension t
- hat may lead to cor pulmonale.
- There is further amplification of the inflammatory response in the airways during exacerbations,
- which may be triggered by bacterial or viral infections or
- by environmental pollutants.
- In general, the inflammatory and structural changes in the airways increase with disease severity and persist on smoking cessation.
PE:
Physical signs of airflow limitation and air trapping (barrel chest, loss of cardiac and liver dullness, prolonged expiration, reduced breath sounds)
Ix
Signs symptoms of exacerbation
noctural cough : why?
- Patients with nocturnal asthma symptoms may have greater nighttime activation of inflammatory cells and mediators, lower levels of epinephrine and increased vagal tone

ddx:
- bronchial asthma
- CHF
-pulmonary edoema,
pneumonia
-bronchiectasis
-pulmonary vascular disease
Outpatient management
- bronchodilators: beta agonist(salbutamol 2.505mg)/ipratropium bromide
- corticosteroids (fev1<50 30mg per day for 1week)
Types of meds
- Acute exacerbation: doxycycline 100mg/ amoxycillin 500mg QID
- CAP: ampicillin_ azithromycin
- severe:
- wihtout beta lactamase inhibitor (piperacillin-tazobactam) /cefepime
- Gram positive:
- staphylo, strepto, pneumococci, tb: cloxacillin, penicillin g,
- gram negative:
- e coli, pseudomonas aeruginosa: aminoglycosides (gentamycin), cephalosporin(ceftriaxone), fluoroquinolones (ciprofloxacin)
- tonsilitis: amoxycillin
- meningitis: ceftriaxon + ampicillin



No comments:
Post a Comment